Anatomy of Peripheral Nerves
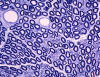
Peripheral nerves consist of fascicles that contain
myelinated and unmyelinated axons. Endoneurium
is the small amount of matrix that is present
between individual axons. The perineurium
is a sheath of special, fiber-like cells that ties
the axons of each fascicle together. Epineurium
is the connective tissue that surrounds the entire
nerve trunk and gives off vascular connective tissue
septa that traverse the nerve and separate fascicles
from one another.
(Click on pictures
to enlarge - click "back" to close)
 |
 |
|
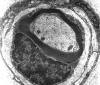
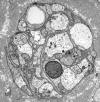
Single myelinated axon
|
Normal nerve
|
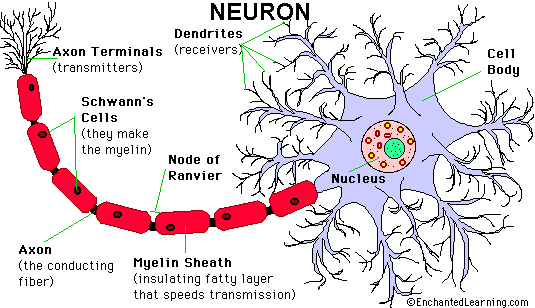
Axons thicker than one micron in the CNS and
peripheral nervous system (PNS) are myelinated. Myelin
is a spiral sheet of cell membrane wrapped around
the axon. In the CNS, myelin is produced by
oligodendroglial cells and in the PNS by Schwann
cells. Each oligodendrocyte makes multiple segments
of myelin that wrap around many axons. Each Schwann
cell makes one segment of myelin. This is one reason
why peripheral myelin regenerates more efficiently. Nodes of Ranvier are points of discontinuity
between adjacent myelin sheaths in which the axon is
not covered by myelin. Unmyelinated axons are
covered by Schwann cell cytoplasm, but there is no
spiraling of Schwann cell membrane around them.
The structure of central and peripheral myelin is
essentially the same. Myelin is composed of 70%
lipids and 30% protein. There are some important
differences in myelin proteins between CNS and PNS.
These differences explain why an allergic reaction
against PNS myelin does not cause central
demyelination and vice versa; and why inherited metabolic disorders of myelin proteins
that affect peripheral nerves do not damage central
myelin. On the other hand, lipids are similar
between PNS and CNS myelin. For this reason,
metabolic disorders of myelin lipids, such as
metachromatic leukodystrophy, affect both, the
central white matter and peripheral nerves.
The myelin sheath acts as an electrical insulator,
preventing short-circuiting between axons. More
important, it facilitates conduction. The nodes of
Ranvier are the only points where the axon is
uncovered by myelin and ions can be exchanged
between it and the extracellular fluid.
Depolarization of the axonal membrane at the nodes
of Ranvier boosts the action potential that is
transmitted along the axon and is the basis of saltatory
(jumping) conduction.
Pathological Patterns of Neuropathy
The pathology of peripheral neuropathy follows three
basic patterns: Wallerian degeneration, distal
axonopathy, and segmental demyelination.
Wallerian degeneration.
The neuronal cell body maintains the axon through
the axoplasmic flow. When an axon is transected, its
distal part, including the myelin sheath, undergoes
a series of changes leading to its complete
structural disintegration and chemical degradation.
|
 |
 |
 |
|
Acute neuropathy
|
Wallerian
degeneration |
Wallerian
degeneration |
Changes
also occur in the neuronal body. The RER
disaggregates and the neuronal body balloons. The
cytoplasm becomes smooth and the nucleus is
displaced toward the periphery of the cell. This
process is called central chromatolysis and reflects activation of protein synthesis in
order to regenerate the axon. Cytoskeletal proteins
and other materials flow down the axon. The proximal
stump elongates at a rate of 1 to 3 mm per day.
Schwann cells distal to the transection also
proliferate and make new myelin.
 |
 |
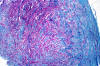 |
|
Lipid material in acute neuropathy
|
Axonal sprouts
|
Traumatic neuroma
|
The degree of regeneration and recovery depends on
how well the cut ends are put together and on the
extent of soft tissue injury and scarring around the
area of transection. If reconstruction is not good,
a haphazard proliferation of collagen, Schwann cell
processes, and axonal sprouts fill the gap, forming
a traumatic neuroma. Wallerian degeneration
was initially described in experimental axotomy.
Neuropathies characterized by Wallerian degeneration
include those that are caused by trauma, infarction
of peripheral nerve (diabetic mononeuropathy,
vasculitis) and neoplastic infiltration.
In distal axonopathy, degeneration of axon
and myelin develops first in the most distal parts
of the axon and, if the abnormality persists, the
axon "dies back". This causes a characteristic
distal ("stocking-glove") sensory loss and weakness. Neurofilaments and organelles accumulate in the
degenerating axon (probably due to stagnation of
axoplasmic flow). Eventually the axon becomes
atrophic and breaks down. Severe distal axonopathy
resembles Wallerian degeneration. At an advanced
stage, there is loss of myelinated axons. Many
clinically important neuropathies caused by drugs
and industrial poisons such as pesticides,
acrylamide, organic phosphates, and industrial
solvents are characterized by distal axonopathy.
Distal axonopathy is thought to be caused by
pathology of the neuronal body resulting in its
inability to keep up with the metabolic demands of
the axon. This explains why the disease begins in
the most distal parts of nerves, and large axons
that have the highest metabolic and nutritional
demands are more severely affected. However, this
question is not settled. It is hard to imagine how
the relatively miniscule neuronal body can keep up
with the metabolic demands of the enormous mass of
the axon. Furthermore, the neuronal body is just as
dependent on the distal axon and its synapses for
trophic interactions that keep it alive and
functioning.
|
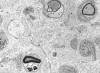 |
|
Demyelinative neuropathy
|
| |
Segmental demyelination,
originally described in experimental lead poisoning,
is characterized by breakdown and loss of myelin
over a few segments. The axon remains intact and
there is no change in the neuronal body. The loss
of saltatory conduction that results from
segmental demyelination leads to decrease of
conduction velocity and conduction block.
Deficits develop rapidly but are reversible because
Schwann cells make new myelin. However, in many
cases, demyelination leads to loss of axons and
permanent deficits. The nerve, in segmental
demyelination, shows demyelinated axons,
thin-regenerating-myelin, "onion bulbs"(see below)
and, in severe cases, loss of axons. The status of
myelin can be evaluated with teased fiber
preparations of peripheral nerves and by
electron microscopy. Neuropathies characterized by
segmental demyelination include acute and chronic inflammatory demyelinative neuropathies,
diphtheritic neuropathy, metachromatic
leukodystrophy and Charcot-Marie-Tooth
disease.
|
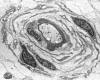 |
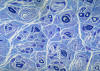 |
|
Hypertrophic neuropathy
|
Hypertrophic neuropathy
|
| |
|
| |
|
| |
|
" Onion bulb" formations
are concentric layers of Schwann cell processes and
collagen around an axon. This proliferation is
caused by repetitive segmental demyelination and
regeneration of myelin and can cause gross
thickening of peripheral nerves (hypertrophic
neuropathy). The central axon is often
demyelinated or has a thin layer of myelin. Onion
bulb formations are the histological hallmark of Charcot-Marie-Tooth disease, but are also seen
in other hereditary neuropathies (Dejerine-Sottas
disease, Refsum disease), in diabetic neuropathy,
and in chronic inflammatory demyelinative
neuropathy.
The pathology of peripheral neuropathy is reflected
in the spinal cord. Acute axonal neuropathy causes
cental chromatolysis. Axonal neuropathy and distal
axonopathy involving the bipolar neurons of the
dorsal root ganglia cause degeneration of the
central axons of these neurons in the gracile and
cuneate tracts of the spinal cord. This lesion is
associated with loss of position and vibration sense
and sensory
ataxia. Neuropathies can be
classified on the basis of their pathological
changes into axonal (Wallerian degeneration and
distal axonopathy), demyelinative, or mixed.
Approach for the Investigation of Peripheral
Neuropathy
The goal of the investigation of peripheral
neuropathy is to establish the diagnosis of
peripheral neuropathy, determine if it is an axonal
or demyelinative process, and find its cause.
Clinically,
neuropathy causes weakness and atrophy of
muscle, loss of sensation or altered sensation
(pain, paresthesias), and weak or absent tendon
reflexes. Nerve conduction studies can
distinguish demyelinative neuropathy (slowing of
conduction velocity or conduction block) from axonal
neuropathy (low-action potential amplitudes). Electromyography (EMG) can distinguish
denervation atrophy from primary muscle disease. CSF examination is helpful, especially in
inflammatory demyelinative neuropathies. Because
cranial and spinal roots bathe in CSF, demyelinative
neuropathies that involve roots cause elevation of
CSF protein. Also, inflammation in nerve roots
causes CSF pleocytosis. Careful history taking with
attention to family history, environmental exposure,
and systemic illness, combined with neurological
examination and laboratory studies can determine the
etiology in most peripheral neuropathies. When the
diagnosis is in doubt, a nerve biopsy studied
by light microscopy, electron microscopy,
morphometry, and teased fiber preparations can give
more definitive information. The sural nerve is
usually chosen for biopsy because it is superficial
and easy to find and it is predominantly sensory.
Sural nerve biopsy leaves a patch of hypesthesia in
the lateral aspect of the foot that is usually well
tolerated.
Diabetic and other neuropathies affect predominantly
small myelinated and unmyelinated fibers that convey
pain and temperature sensation. Degeneration in
these "small fiber neuropathies" involves the
most distal portions of nerve fibers that are found
in different organs and tissues (somatic fibers)
rather than fibers in major nerves. Nerve conduction
studies and EMG in such cases may be normal and the
sural nerve biopsy may be difficult to interpret.
The diagnosis can be made with a skin biopsy.
A 3-4 mm plug of skin is removed with a punch and
sectioned with a microtome. The sections are treated
with antibodies to Protein Gene Product 9.5 which
reveal small nerve fibers that penetrate the
epidermis. The density of these fibers is reduced in
small fiber neuropathies.
|
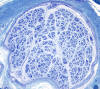 |
|
End stage axonal neuropathy
|
T he pathological changes of most peripheral
neuropathies (axonal degeneration, segmental demyelination or a combination of these) are not
specific. In any active neuropathy, there are
macrophages removing myelin and axon debris.
Advanced axonal neuropathy shows loss of myelinated
axons and increased endoneurial collagen. Some
chronic demyelinative neuropathies show hypertrophic
changes. Thus, in most neuropathies, the sural nerve
biopsy can only establish the diagnosis of
neuropathy and distinguish axonal from demyelinative
and acute from chronic neuropathy, but cannot
determine the cause of neuropathy. Only a few
peripheral neuropathies show disease-specific
pathological changes allowing a specific diagnosis.
These neuropathies include acute and chronic
inflammatory demyelinative neuropathies, hereditary
motor and sensory neuropathies, vasculitis, sarcoid
neuropathy, leprosy, amyloid neuropathy, neoplastic
invasion of peripheral nerves, metachromatic
leukodystrophy, adrenomyeloneuropathy, and giant
axonal neuropathy.
Principal CAUSES of Peripheral Neuropathy
- 1. Autoimmunity
(inflammatory demyelinative
polyradiculoneuropathies).
- 2. Vasculitis
(connective tissue diseases).
- 3. Systemic
illness (diabetes, uremia, sarcoidosis, myxedema,
acromegaly).
- 4. Cancer (paraneoplastic
neuropathy).
- 5. Infections
(leprosy, lyme disease, AIDS, herpes zoster).
- 6. Dysproteinemia
(myeloma, cryoglobulinemia).
- 7. Nutritional
deficiencies and alcoholism.
- 8. Compression and
trauma.
- 9. Toxic
industrial agents and drugs.
- 10. Inherited
neuropathies.
-
Diabetic Neuropathy
|
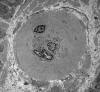 |
|
Arteriole in diabetic nerve
|
| |
| |
The most common cause of neuropathy in clinical
practice is diabetes. Peripheral neuropathy develops
in more than half of long term diabetics. Diabetes
causes several types of neuropathy, which include
chronic symmetrical polyneuropathy, proximal
neuropathy (diabetic amyotrophy), mononeuropathies,
and cranial radiculopathies. The pathogenesis of
diabetic neuropathies is poorly understood. Many of
them have an ischemic basis. A prominent finding in
diabetic neuropathy is thickening of arterioles due
to increased deposition of basement membrane
material, similar to changes that occur in brain
arterioles and glomerular capillaries. Nonenzymatic
glycation of neural structures and other biochemical
changes in diabetes probably play a role also.
Inflammatory Demyelinative Neuropathies
These uncommon neuropathies are presumed to be
immune disorders in which antibodies and activated
T-lymphocytes, reacting with antigens present on
peripheral nerves, elicit an inflammatory and
macrophage reaction that destroys myelin and axons.
The strongest evidence of a humoral immune reaction
in these neuropathies is that plasma exchange
results in significant clinical improvement. The
participation of cellular immunity is underlined by
the pesence of T-lymphocytes around blood vessels in
affected nerves. The two main entities in this group
are the Guillain-Barré syndrome and chronic inflammatory demyelinative neuropathy.
An experimental model of demyelinative neuropathy, experimental allergic neuritis (EAN), can be
produced by injecting animals with myelin and Freund
adjuvant or purified peripheral myelin protein P2.
EAN is a cell-mediated immune reaction.
|
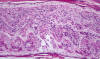 |
|
Guillain-Barre syndrome
|
|
|
The Guillain-Barré Syndrome(GBS)
is not a single disease entity. It includes several
variants: Acute inflammatory demyelinative
polyneuropathy (AIDP), acute motor axonal neuropathy
(AMAN), and the Miller-Fisher syndrome (MFS). AIDP
accounts for 90% of GBS. It begins with paresthesias
in the toes and fingertips, followed by rapidly
advancing weakness and areflexia. Weakness reaches a
plateau within four weeks, after which recovery
begins. Some cases are fulminant, evolving in one or
two days. At the height of their disease, many
patients are completely
paralyzed and unable to
breathe. Even with modern intensive care,
approximately 5% of patients die from respiratory
paralysis, cardiac arrest (probably due to autonomic
dysfunction), sepsis, and other complications. Ten
percent of those who recover have residual weakness.
Though easy to diagnose in its classical form, GBS
is often missed because of atypical clinical
features which include ophthalmoplegia, ataxia,
sensory loss, and dysautonomia. Plasma exchange
(presumably removing the offending antibodies) and
intravenous immunoglobulin are the treatments of
choice. The two key laboratory abnormalities in GBS
are decreased nerve conduction velocity
or conduction block and elevated CSF protein
with relatively few cells (albuminocytologic
dissociation).
Peripheral nerves show perivenular mononuclear
cells, demyelination (myelin proteins are the source
of elevated CSF protein), and macrophages. Axonal
damage, which accounts for the permanent deficits,
is variable and may be severe. The pathology is most
severe in spinal roots and plexuses and less
pronounced in more distal nerves. In the phase of
recovery, the nerve contains thin myelin sheaths,
indicating myelin regeneration. AMAN shows axonal
damage with little inflammation.
About 20% to 30% of GBS cases are preceded by an
infection with Campylobacter
Jejuni. An equal number are preceded
by Cytomegalovirus (CMV) infection. The rest are
preceded by Mycoplasma and other infections, or
vaccinations. The bacterial wall of C. jejuni
contains GM1 ganglioside. Anti-ganglioside
antibodies, generated in the course of the
infection, cross-react with GM1 ganglioside present
in the axonal membrane at the nodes of Ranvier and
in paranodal myelin. This contact elicits
inflammation that damages these structures. Anti-GM1
antibodies are found in the serum of GBS patients.
GBS following CMV infections has anti-GM2
antibodies.
Chronic inflammatory demyelinative
polyradiculoneuropathy (CIDP)
follows a chronic or relapsing course over many
months or years and may cause severe permanent
disability. Nerve conduction studies show decreased
conduction velocity, conduction block, and prolonged
distal latencies and F waves. In the active phase of
the disease, the CSF shows elevated protein without
increased cells. Pathologically, peripheral nerves
show demyelination, thin (incompletely regenerated)
myelin, and hypertrophic changes due to recurrent
attacks of demyelination with intervals of repair.
In chronic cases, there is significant axonal loss.
Inflammation is variable, sometimes absent. The
pathology is most severe in proximal nerve segments
and spinal roots and may not be full blown in the
sural nerve biopsy. CIDP is thought to represent an
autoimmune T-cell and antibody reaction against
unknown myelin antigens. Its treatment consists of
plasma exchange, intravenous immunoglobulin, and
corticosteroids.
The GBS and CIDP are the counterparts of MS for the
peripheral nervous system. They are important,
because timely intervention with plasma exchange can
prevent death in the GBS and severe permanent
disability in CIDP. There are standardized criteria
for their diagnosis, based on the clinical, CSF,
nerve conduction, and biopsy findings.
Hereditary Neuropathies
The inherited neuropathies are rare as a group and
include lysosomal storage diseases,
peroxisomal disorders, and familial amyloidoses.
Neuropathy, in these diseases, is a component of a
systemic metabolic defect. The inherited
neuropathies include also a group of diseases called hereditary motor and sensory neuropathies, in
which neuropathy is the main or only abnormality.
The most common entity in this group and the most
common overall familial neuropathy is Charcot-Marie-Tooth
disease.
Charcot-Marie-Tooth disease
(CMT) is not a single entity but a group of
inherited neuropathies that are divided into 3
phenotypes, CMT1, CMT2, and X-linked CMT. CMT1 is
the most common inherited peripheral neuropathy. It
involves 1 in 2500 persons and is autosomal
dominant. It causes weakness and atrophy of distal
muscles, especially those innervated by the peroneal
nerve ("stork leg"), pes cavus, sensory loss, and
action tremor in some patients. It begins in
childhood or adolescence and progresses slowly,
involving other nerves. It is compatible with a
normal lifespan. Nerve conduction studies show
decreased conduction velocity. The nerve biopsy in
CMT1 shows demyelination, myelin regeneration (thin
myelin), axonal loss, and onion bulbs. In
longstanding cases there is gross thickening of
nerves, hence the term hypetrophic
neuropathy.
CMT1 is genetically diverse. Its most common form is
due to duplication of a segment of
chromosome 17 (17p11.2-p12) that contains the gene
for a 22 kd peripheral myelin protein, PMP22.
This protein probably also plays a role in Schwann
cell differentiation. CMT1 patients have three
copies of the normal gene and presumably produce 1.5
times as much PMP22 as normal people do. Other forms
of CMT1 are caused by mutations of the PMP22 gene or
mutations of the Myelin Protein Zero (MPZ) gene.
CMT2 is a distal axonopathy with a diverse genetic
background. X-linked CMT is caused by mutation of a
gap junction protein, connexin 32. A deletion of the
PMP22 gene causes hereditary neuropathy with
pressure palsies. Autosomal dominant and autosomal
recessive mutations of PMP22, MPZ, and other genes
cause CMT3 (Dejerine-Sottas
disease), a severe infantile demyelinative
hypertrophic neuropathy. These molecular
abnormalities underline the importance of myelin
proteins for structural stability of myelin and show
how diverse genetic abnormalities can cause a
similar phenotype.
|
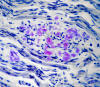 |
|
Amyloid neuropathy
|
| |
| |
Familial amyloid neuropathies
(FAP) are a group of familial systemic amyloidoses
with involvement of peripheral nerves. The most
common FAP is caused by an autosomal dominant
mutation of the transthyretin
gene on 18q11. The mutant protein is deposited in
the form of amyloid and damages peripheral nerves,
the heart, kidneys, gastrointestinal tract, and
other organs. In nerves, amyloid damages first and
most severely small fibers, causing loss of pain and
temperature sensation and autonomic dysfunction.
Transthyretin is produced in the liver. Liver
transplantation arrests the progression of the
disease.
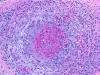
Vasculitic Neuropathy
|
 |
|
Necrotizing arteritis
|
| |
| |
| |
| |
| |
Polyarteritis nodosa
and other vasculitides often involve peripheral
nerves causing single or multiple mononeuropathies
(due to nerve ischemia), asymmetric polyneuropathy,
and distal symmetric polyneuropathy. A sural nerve
biopsy along with a muscle biopsy are the best
tissues for establishing the diagnosis of vasculitis.
The nerve biopsy is diagnostic in over half of
patients with systemic vasculitis and clinical
neuropathy, and the diagnostic yield increases with
the addition of a muscle biopsy. Such biopsies show
necrotizing arteritis, perivascular inflammatory
infiltrates, hemorrhage and hemosiderin deposition,
neovascularization in epineurial arteries, and
variable changes in nerve fascicles, depending on
the severity and stage of neuropathy. The muscle
shows vasculitis and denervation atrophy.
Further reading from source:
( www.neuropathology-web.org
)
Lauria G, Lombardi R. "Skin biopsy: a
new tool for diagnosing peripheral neuropathy."
BMJ
2007;334:1159-62
|
Do
you or a loved one suffer from
peripheral neuropathy?
-
 The road to recovery STARTS with
the ReBuilder™! The road to recovery STARTS with
the ReBuilder™!
- Aggressive Neuro-stimulus: The ReBuilder™
This amazing device is the
closest thing to a cure you can
find.
The ReBuilder’s patented electrical signal device has been proven
94% effective in clinical studies in reducing painful symptoms of neuropathy.
FDA Approved ♦ Covered by Most
Plans with a
Prescription

|
Back to ReBuilder Page
.png)

The ReBuilder 2407
|